Medical breakthrough: Doctors transplant a genetically modified pig heart into a man
Published 9:30 am Friday, February 24, 2023
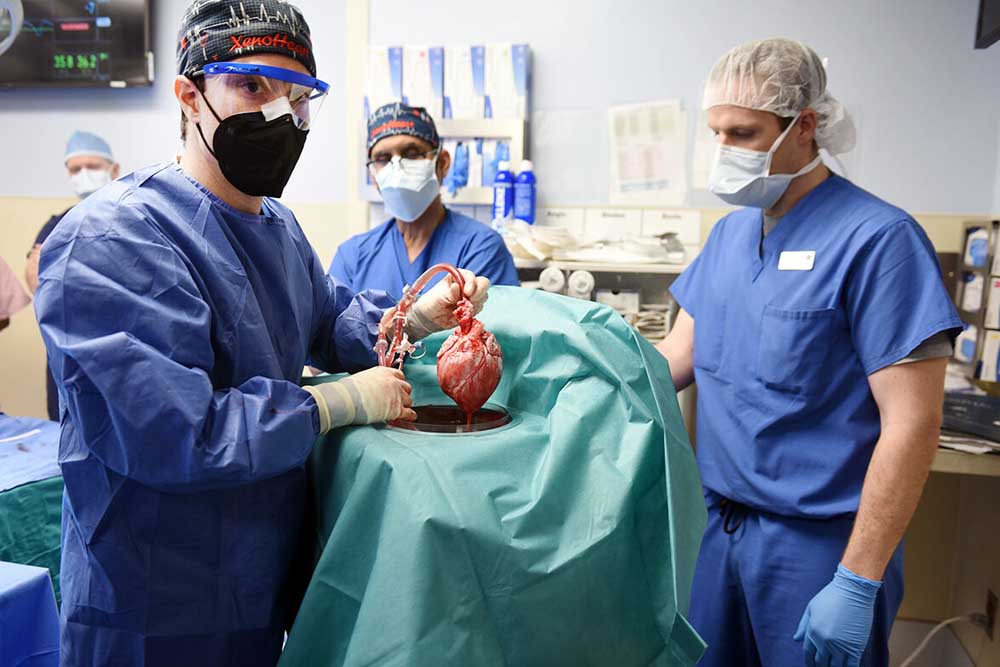
- Members of the University of Maryland School of Medicine surgical team lift the genetically modified pig’s heart out of a perfusion device, which preserves the donor heart at a fixed temperature while continuously pumping an oxygenated solution through the organ, as part of the first successful pig-to-human heart transplant in January 2022.
BALTIMORE, Md. — A pig was at the heart of a medical breakthrough last year when doctors at the University of Maryland Medical Center performed the first-ever pig-to-human heart transplant.
The pioneering patient, 57-year-old David Bennett, had terminal heart disease but was unable to get a human heart transplant. Instead, he received a genetically modified pig’s heart on Jan. 7, 2022. With the new heart beating in his chest, he lived for two more months.
Though the use of heart valves from pigs and cows is commonplace in human heart surgeries today, researchers have been working on pig-to-human heart transplant techniques for more than 30 years.
Bennett’s transplant was a landmark medical breakthrough. The American Heart Association says such transplants may one day become commonplace.
“I think that’s probably the next quantum step in medicine,” said Dan Fitzsimons, assistant professor in the Department of Animal, Veterinary and Food Sciences at the University of Idaho. He studies heart functions in mammals.
Landmark accomplishment
Even though Bennett didn’t survive long, his heart transplant was a “pioneering, landmark accomplishment,” said Dr. Paul Wang, director of the Stanford Cardiac Arrhythmia Service and professor of medicine and bioengineering at Stanford University.
Wang and other doctors hope that xenotransplantation — grafting or transplanting organs or tissues between species — will become the norm, Wang said.
“That could eventually save many, many lives, and improve quality of life,” he said.
The biggest limitation is the human body’s rejection of an organ from an animal.
Rejection in a human-to-human transplant is much more understood and largely preventable, Wang said.
More research is needed, he said.
“We have to understand better the mechanisms of rejection — how the immune system works in this kind of situation,” he said. “Once we do that, I’m certain we’ll develop better ways to result in better outcomes.”
In Bennett’s case, the biggest breakthrough was genetic modification of the pig’s heart, reducing the likelihood of a rejection response, Wang said.
“That alone was a very big advance,” he said. “However, clearly, more has to be done and more has to be learned about how to do that. But we’re clearly in a position we’ve never been in before, because we know so much more than we did about these processes.”
The Food and Drug Administration has not approved the use of pig hearts in human transplants. The agency made a one-time “compassionate use” exception, as Bennett was ineligible for a human heart transplant.
Doctors are encouraged, but there’s a long way to go, Wang said.
Heart differences
Doctors expected the genetically modified pig heart to behave the same as pig hearts usually do, said Dr. Timm Dickfeld, professor of medicine and director of electrophysiology research at the University of Maryland School of Medicine.
They anticipated the heart would beat faster than a human heart, but the actual intervals were longer than those of a human heart, he said.
The researchers are currently looking into several possible explanations.
The study will help the medical field navigate the new complexities, he said.
“It helps us to understand what to watch out for in the future, and to detect and address any potential changes that could at some point be harmful to a patient,” he said. “This will allow us to get better with each patient, and to come close to make xenotransplantation a standard way of caring for our sick patients.”
The waiting list
The need for heart transplants is great. Nearly 3,400 patients are on the waiting list for a heart transplant, according to the United Network for Organ Sharing.
About 20% of those patients die while waiting for a transplant or become too sick to undergo the complex procedure, according to the Heart Association.
Currently, the process involves waiting for a healthy human to die so the heart becomes available, said Fitzsimons, the Idaho professor.
The potential use of animal hearts is “a game-changer for any single person that’s on transplant lists,” he said. “All of a sudden, you’re not waiting for that identical human match.”
A pig heart could be used as a place-holder of sorts, extending a patient’s life while waiting for a human heart. Or it could become a permanent replacement.
“It could be used in transition, but I think the contribution and advancement to help humans would be very large if it could be an eventual solution for people,” Wang said. “That’s really what’s necessary to change people’s lives.”
Genetically modified pigs
The genetically modified pig heart donor in the University of Maryland surgery came from Revivicor Inc., based in Blacksburg, Va.
The company wants to provide organs from gene-edited pigs to address the shortage of human organs for transplant.
Part of Revivicor’s work centers on what happens when a lone star tick bites a person. The bite transfers a sugar molecule called alpha-gal into the body. In some people, this causes mild to severe allergic reactions to red meat, such as beef, pork or lamb, or reactions to other foods that come from mammals, such as dairy products or gelatins.
This allergy is known as alpha-gal syndrome, according to the Mayo Clinic.
Revivicor has developed two types of pigs, said Dewey Steadman, a company spokesman:
• Gal-Safe pigs, which have had the alpha-gal gene removed. This helps prevent a human recipient from rejecting the pig’s heart.
• Ten-gene edited pigs, in which the alpha-gal gene and three other porcine genes were silenced, and six human genes are added to reduce the chances a human host would reject a pig heart.
The pigs are identical to those found on farms around the U.S., he said.
Gal-Safe pigs have been approved by FDA for medicinal use and human consumption.
Over the past 18 months, Revivicor has given surplus Gal-Safe meat to patients who suffer from alpha-gal syndrome, Steadman said. Ten-gene pigs are not yet approved for this use.
Revivicor’s pigs are raised in a “high herd health environment,” where workers change clothes and “shower-in” to keep the pigs free of pathogens.
“This isn’t very different from commercial pig barrier facilities,” Steadman said. “However, the list of infectious disease agents to exclude goes beyond those pathogens of risk to pigs to include agents that might be passed from pigs to humans in the context of an organ transplant.”
The company recognizes the potential for Gal-Safe pigs in other uses, such as providing heart valves, “but we’re focused on addressing the shortage of tolerable, transplantable organs, especially kidneys and hearts,” Steadman said.
Revivicor is working with the FDA to develop a clinical path forward and move into human studies.
‘Considerable’ challenges
Pigs have emerged as the most promising donor species due to their similar heart size and anatomy, the feasibility of precise genetic modifications, favorable breeding characteristics and relatively low risk of infection, according to an article in the Journal of the American College of Cardiology last July.
The authors — Jacinthe Boulet, Jonathan Cunningham and Mandeep Mehra — could not be reached for comment.
But challenges remain, they wrote. Pigs and humans have different blood pressures and body temperatures.
It’s also uncertain whether a genetically engineered pig heart will adapt to cardiometabolic stress during effort and exercise, or whether a change of blood flow orientation to the lungs from a horizontal to an upright position matters, they said.
They predicted uniform social acceptance of the practice is “unlikely.”
“Major differences in cultural and religious beliefs will make it particularly challenging to build consensus guidelines,” the authors wrote, adding that other potential ethical considerations include “animal rights” and the “view of putting humans above all other animal species.”
“These considerable ethical, social and legal challenges will require careful consideration and discourse in a manner concurrent with scientific advances in the field,” the authors wrote.
FDA’s position
“Protecting patient health and safety is our highest priority,” said Carly Kempler, press officer for the FDA. “The FDA recognizes that the use of xenotransplantation products show great promise, but there are also potential risks. Our goal is to spur innovation and efficient access to potentially transformative products, while ensuring safety.”
Applications for clinical studies involving the use of pig hearts in human volunteers are evaluated on a case-by-case basis, Kempler said.
The FDA assesses whether proposed studies are “reasonable and acceptable” in light of the potential benefits that may be provided to patients.
Because of the potentially serious public health risks of possible infections spread between people and animals, the FDA recommends that xenotransplantation be limited to patients with serious or life-threatening diseases for whom adequately safe and effective alternative therapies are not available.
“Candidates should be limited to those patients who have no other viable choices, and have the potential for a clinically significant improvement with increased quality of life following the procedure,” Kempler said.
Heart valves
About 65,000 heart valve replacements are performed every year in the United States, according to the website HeartValveSurgery.com, an educational resource for patients. Patients and their medical teams choose whether to use a biological or mechanical valve to replace the diseased valve.
Biological valves are fashioned from pig, cow, horse or donated human tissue. The valves are then treated to mitigate the need for anti-rejection medications.
Hearts are harvested from freshly killed pigs, according to the website. These pigs are not grown specifically for harvesting of their hearts but are raised for human consumption.
Unlike a pig valve replacement, a cow valve uses tissue from the cow’s heart — not the actual heart valve. The cow valve is too large compared to a human heart valve and would not fit in a human heart.
Pig and cow heart valves are frequently used, Wang said. The nature of the tissue is suitable for long-lasting results, he said.
Pig heart valves last an average of 10 years, with some lasting up to 17 years.
Fitzsimons studies the regulation of cardiac contraction and relaxation in mammalian hearts — pigs, cows and rodents — on the UI campus in Moscow.
Valves are a critical part of heart function, he said.
“The valve is like a door — you want the door closed when it’s closed and open when it’s open,” he said. “You don’t want the front door of your house just opening when it wants to or closing when it wants to. You want to regulate who comes in and out of your house, and it’s the same thing for your heart.”
The need for pig and cow heart valves isn’t going away, particularly as diagnostic tools and surgical skillsets improve, Fitzsimons said.
“Valve replacement really is a game-changer for a lot of people,” he said. “They can recapitulate near-normal, if not normal, cardiac function, which means they’re able to do pretty much everything. That’s massive.”
Even with advances in artificial and mechanical valves, pig and cow hearts will likely continue to play an important role, Wang said.
Outlook for the future
“We are at least a decade away from xenotransplants becoming a standard option for patients to help address the organ shortage,” said Dickfeld, the electrophysiology researcher. “We still have to conduct pre-clinical studies and then get permission from the FDA to start clinical trials, which will likely take a few years to conduct.”
Farmers stand to play “an amazing role” in those advances, said Wang, the Stanford doctor.
“If you can think of the possibility of extending human life in a very important way and improving quality of life, that’s a very exciting thing to be part of,” he said.






